We are dedicated to provide high clinical benefits and financial returns, building innovative companies from the best research teams worldwide.
We gather execution-focused teams to deliver meaningful results.
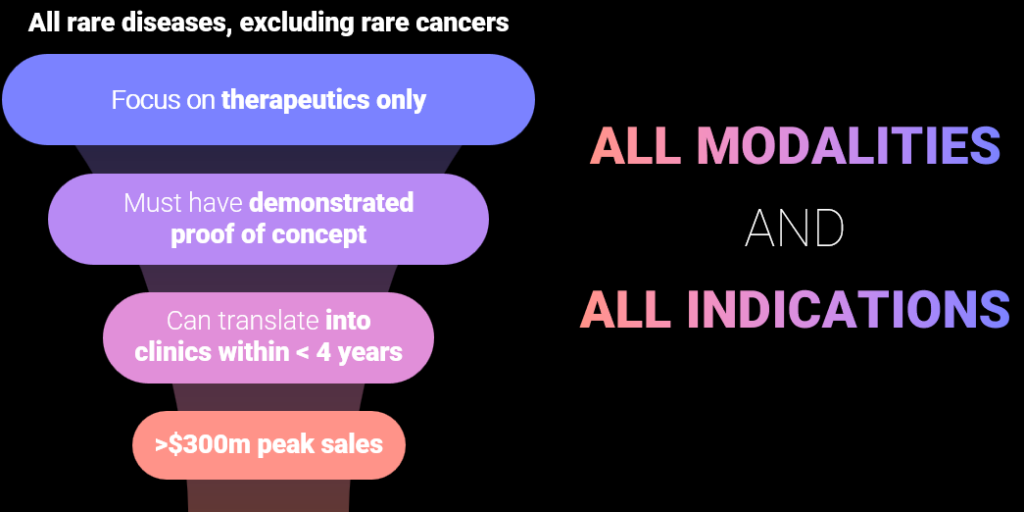
Opportunity in rare disease is real and strong
VALUE over volume
High pricing per patient
Average annual price per patient with rare diseases 218k€ vs 13k€ for common diseases
high success rate
2X likelihood of success
Likelihood of success from Phase 1 to marketing authorization: 17% for orphan drugs vs 8% in general
high ROI
7.2X investment multiple
7.2x average investment multiple from Phase 1 to approval in orphan drugs vs. 2.1x for non-orphan
our investment thesis
OUR LEGACY

A relentless partner to address rare diseases
rare diseases
since 1958
550+ FTEs and 215k+ volunteers
€1.7b invested in R&D for rare diseases
Renown expertise
to develop programs
for rare diseases
Network of 6,000+ medical and scientific experts in rare diseases and across all technology modalities
38 clinical trials on-going or under preparation on 28 different indications
Up to 200 new research projects funded every year
An association with a proven track-record
as venture-builder
Creation of Genosafe in 2003 – CRO for gene and cell therapy
Creation of BIMR in 2013 with BPIfrance Investment fund for rare diseases and innovative therapies – 7 companies supported
Creation of Atamyo in 2020 – Gene therapy biotech for muscular dystrophies
Contributed to the creation of 600+ direct jobs